I can now officially reveal that we are moving the BWJoneslab from the University of Utah, Moran Eye Center to the University of Pittsburgh, Department of Ophthalmology, effective August 1st, 2025.
I’ve been at the University of Utah since 1989 as an undergraduate, so 36 years at this institution and 26 years at the Moran Eye Center where I started as a graduate student. I have earned my bachelors degree here, a PhD, two postdoctoral fellowships in cell cycle biology, and neuroscience, and then my roles as faculty in multiple departments, and leadership on the University of Utah Senate. I have taught undergraduates, graduate students, medical students, and postdoctoral fellows over the years.
I have worked with development to help raise millions of dollars in donations to my department and others. I worked with Greg Jones (no relation) when he was the Governors science advisor to help advocate for the University of Utah. I have appeared in television ads for research here at the University of Utah and the Moran Eye Center.
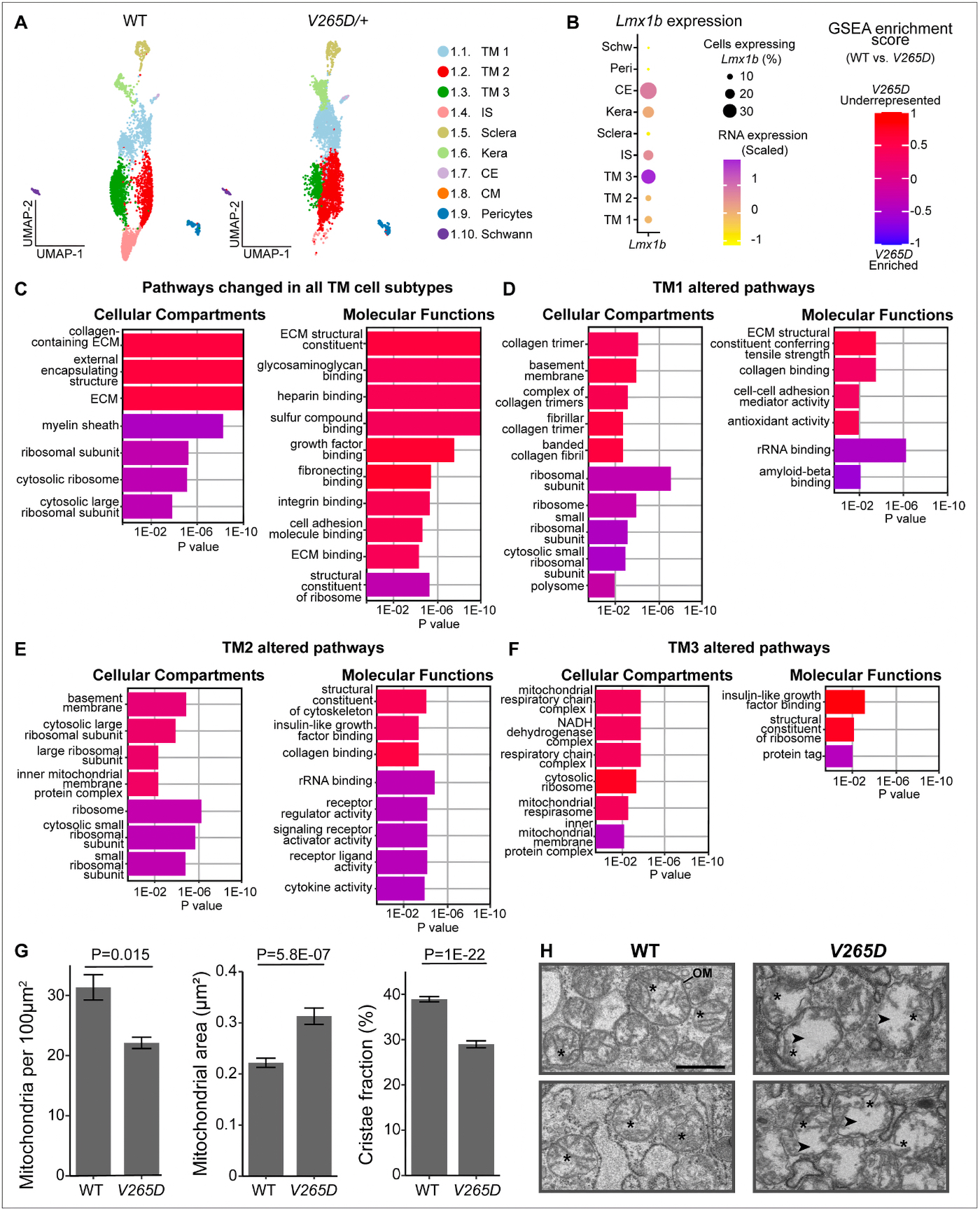
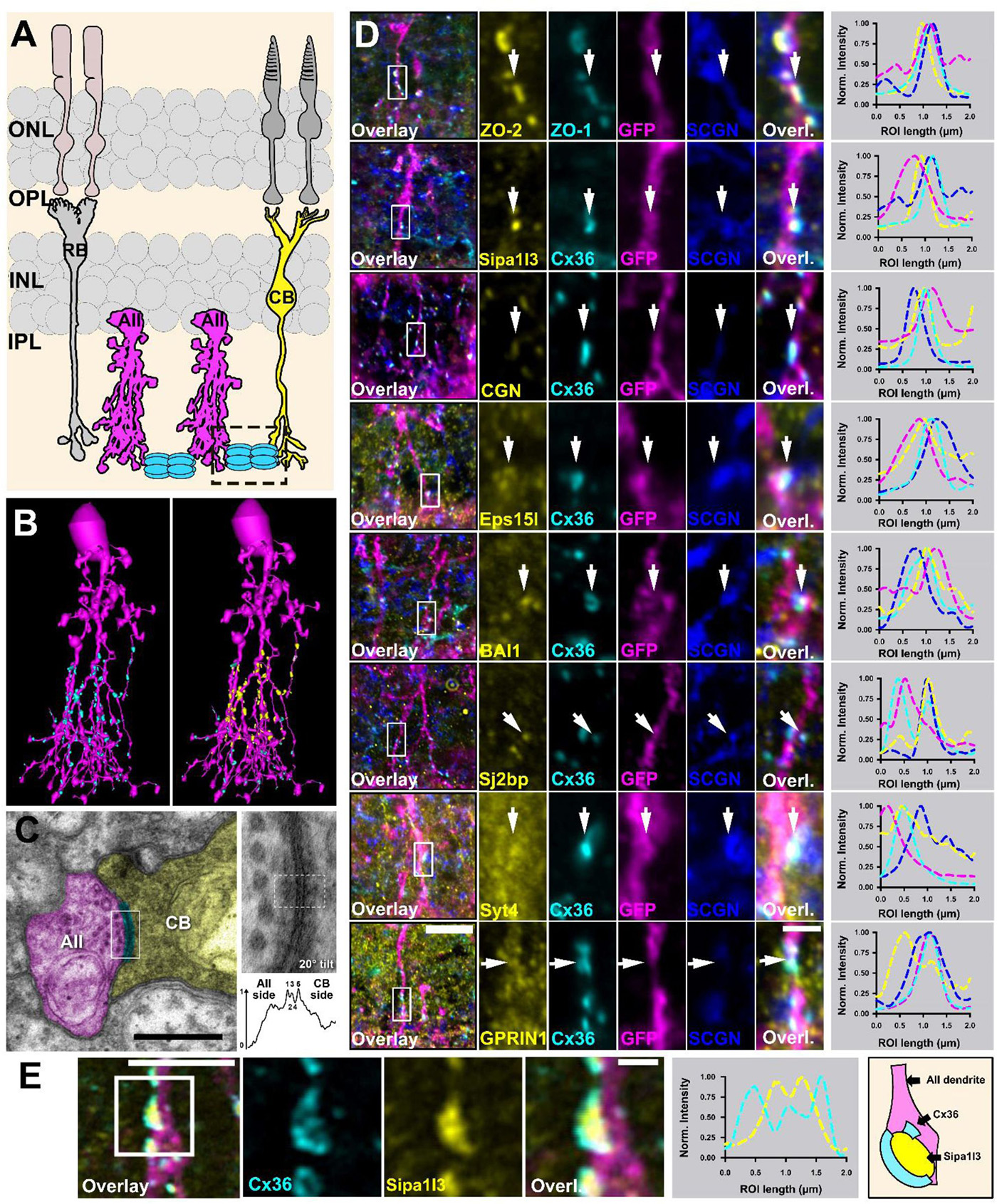
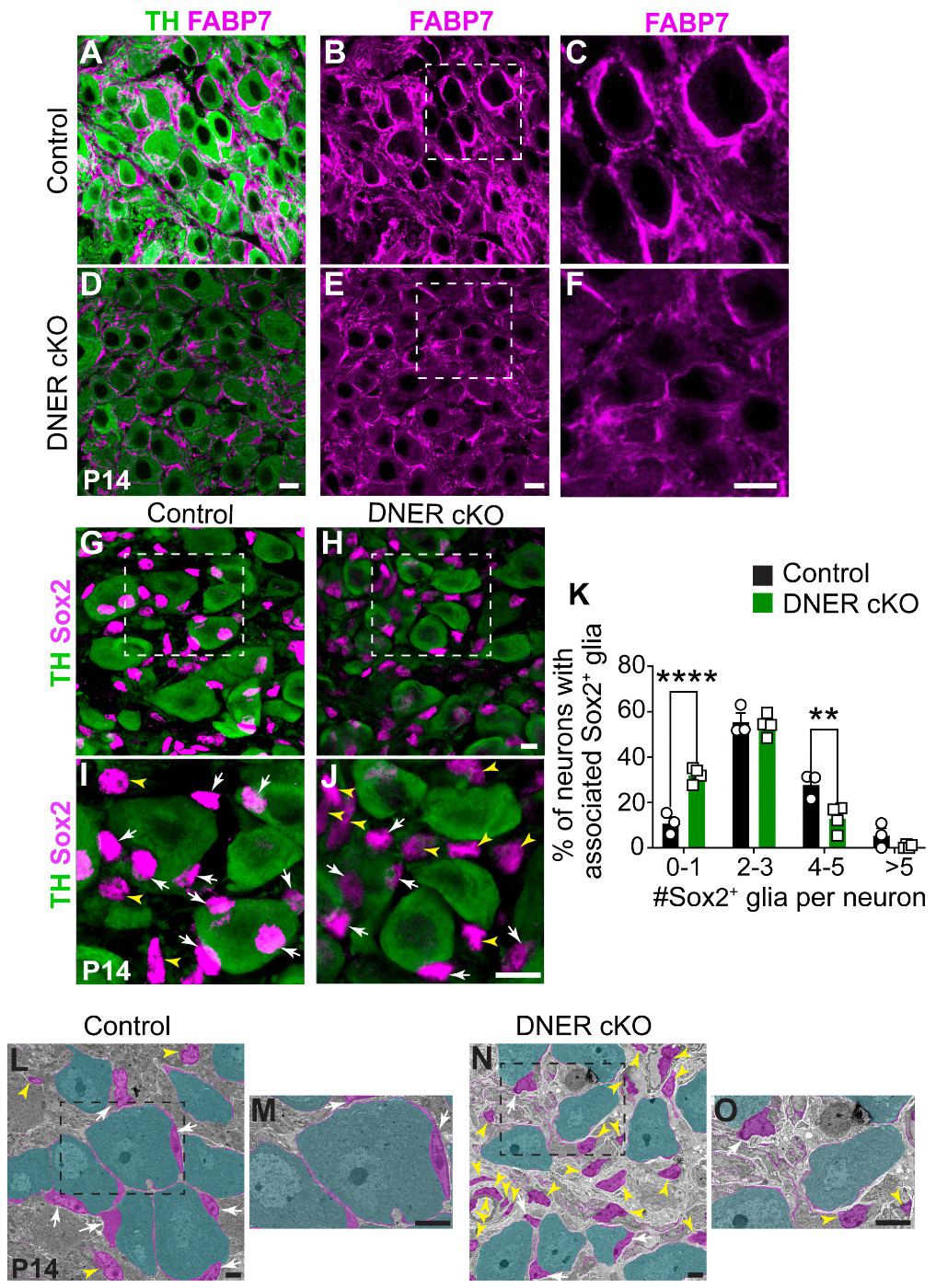
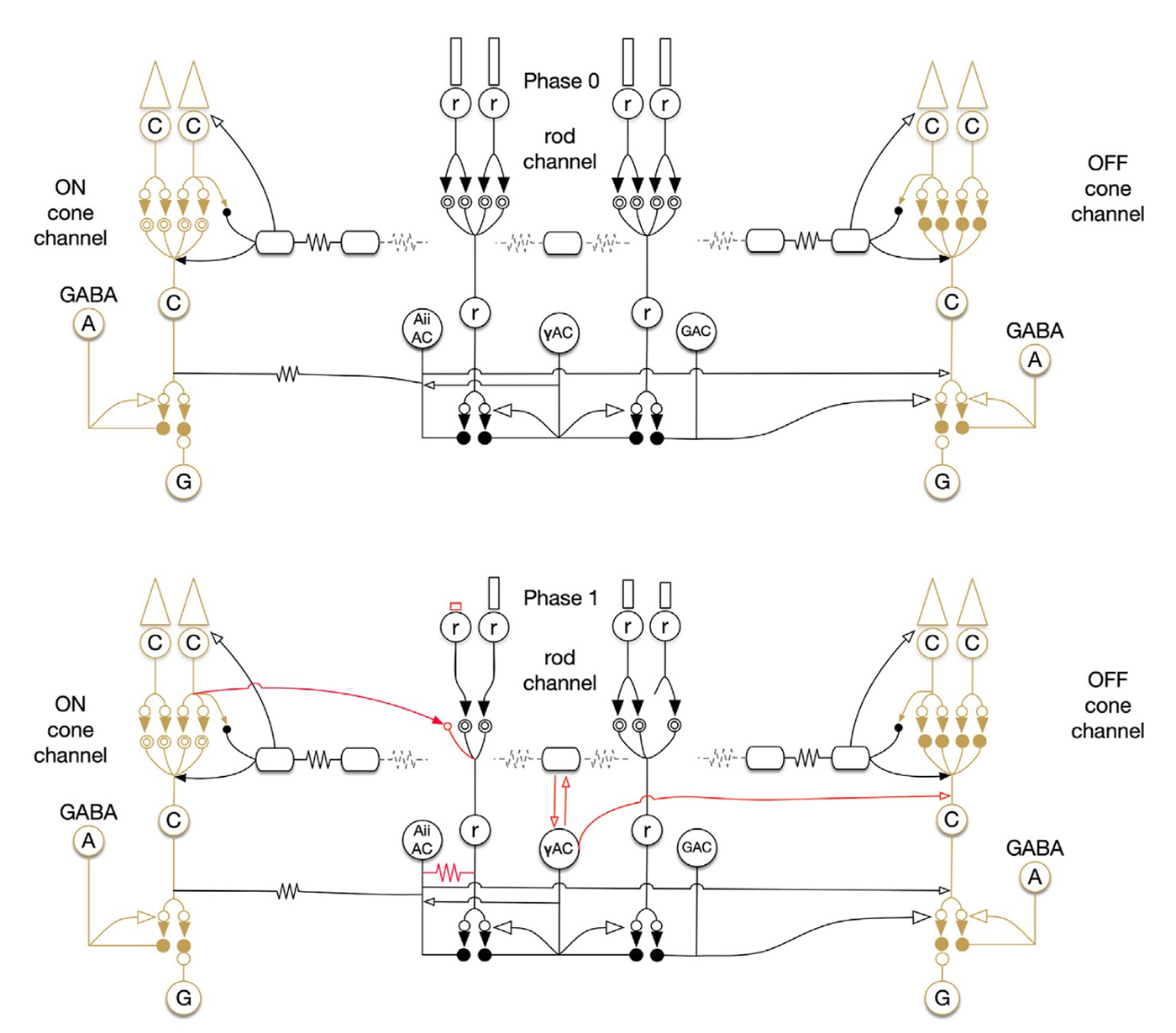
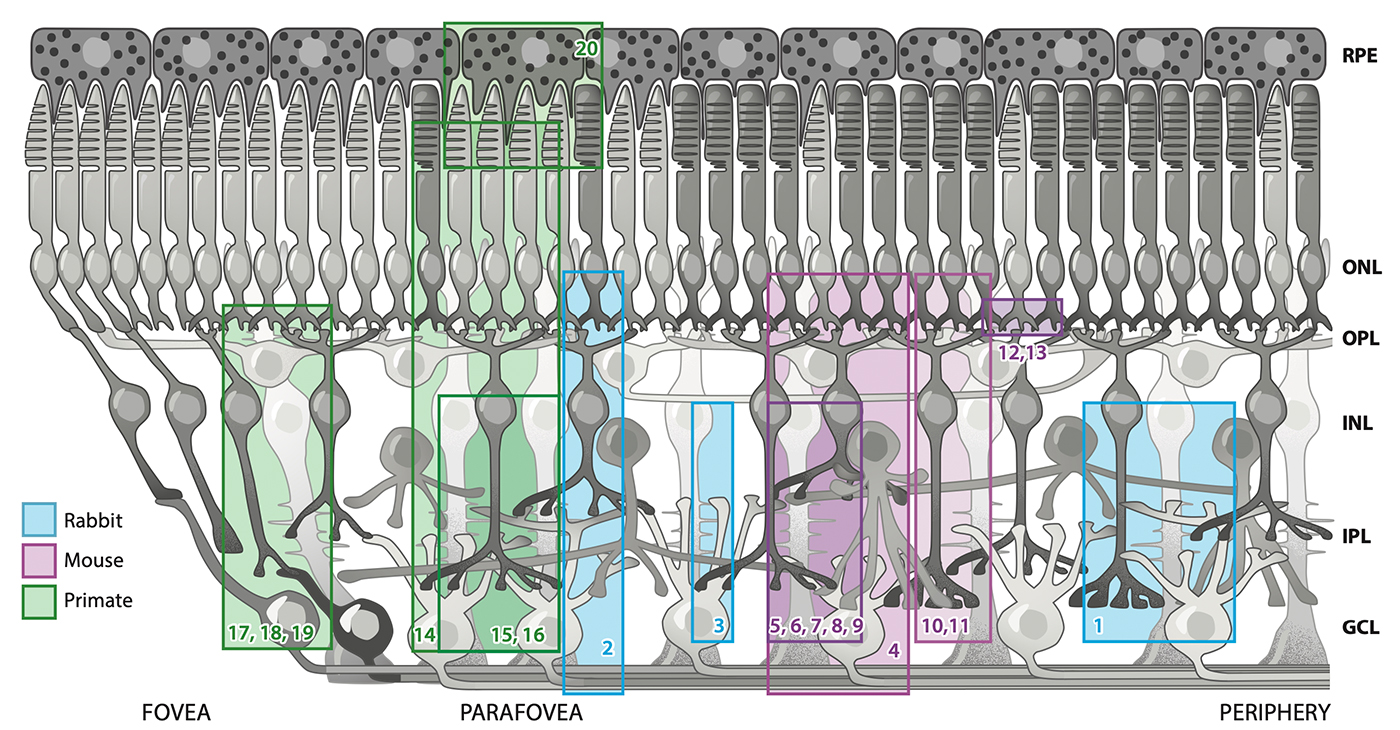
As an undergraduate, I worked with the Biology Department to create some of the world’s first online teaching tools for anatomy and embryology using Hypercard. As an undergrad and then graduate student, I ran some of the first websites and blogs on the Internet from servers here at the University of Utah with the encouragement and blessing of the University of Utah, and have worked with IT over the years to allow this information to be disseminated around the world. I have managed the world’s first online textbook, Webvision for over 25 years that has been hosted on servers in my office or lab, and paid for out of my own pocket. We have modeled Moran CORE on Webvision that is garnering millions of visits per year. We have partnered with the Scientific Computing Institute to create the tools that allowed us to construct the world’s first retinal connectomes. My lab has also created the world’s first pathoconnectomes in any system with software that we have continued to develop, and for many years, we were the only NIH funded laboratory that performed connectomics related work. We have absolutely been global pioneers in this space. This was all possible because the computational environment at the University of Utah allowed and encouraged experimentation and exploration with novel new computational tools.
I will forever be grateful to the people here who helped me along from my chairman, Randy Olson on down. I will absolutely miss folks here, and it will be bittersweet leaving, but the University of Pittsburgh Ophthalmology program is such an amazing opportunity filled with phenomenal people and goals that I could not resist the offer. Every year has brought at least one offer to move the lab to another institution, and it’s been easy to turn down many of those offers, but this move to Pitt was the most compelling yet, filled with amazing colleagues who also happen to be good human beings who are engaged in exciting science. Leadership at Pitt understands that to continue pushing science forward means a commitment to a computational IT environment that is supportive of that work and the role of computational science in it. They are supportive and eager to grow initiatives there that take advantage of those aspects of biomedicine. Housing is far more affordable around Pittsburgh than it is here in Salt Lake City, and the vibe in Pittsburgh is one of excitement, equity, and investment in the diverse people that make up the communities around Pittsburgh. This level of support and commitment to people makes moving the whole lab and all the personnel there much more attractive.
And my vote will count in Pennsylvania.
We will be anchoring histology, ultrastructure and connectomics at Pitt, as well as helping them to hire out the next phase of expansion with sensory neurosciences as a focus. I’m grateful to José-Alain Sahel, and John Ash for their commitment and efforts to bring us on, and to the entire faculty of the Ophthalmology department, the neurosciences and engineering communities at Pitt who will be our colleagues and collaborators on a variety of science projects moving forward to help understand vision and vision rescues. As part of this, Becca will be leaving the BWJoneslab team, and taking a completely independent faculty line job with her own startup package at Pitt, so we’ll still be working collaboratively at the same institution which is verrrrry cool.
On a personal note, 2025 has been chaotic and tumultuous for so many reasons. It has been the most difficult, stressful year of my life with changes desired and very much undesired. This move is a decision that Hilari and I were very much looking forward to as a new adventure for Team Jones, and I am absolutely heartbroken to be making this move without the love of my life and my best friend. She was looking forward to living in a new city, and all of the excitement of starting fresh and discovering new things, new arts, new opportunities for philanthropy and exploration. I don’t know how to even process this as it feels like I’ve lost the part of me that helps understand, plan and contextualize everything. Every move like this has its downsides, but those aspects are the ones that scare me most, and feel the most destabilizing. I miss you, my love. And I will continue to work to make you proud of me.